The use of technologies for CO2 utilization has gained a great deal of attention around the world over the past few years. In the long term, they will be an indispensable component for future economic activity. In this article we show different CO2 conversion and utilization pathways and explain why GIG Karasek is pushing electrochemical CO2 conversion in various research projects.
1. Why GIG Karasek is focusing on CO2 utilization
Carbon capture and utilization (CCU) refers to technologies that capture CO2 from the atmosphere or flue gases and subsequently convert it into value-added products such as plastics, building materials, synthetic fuels or chemicals (e.g. carbon monoxide, methanol, ethanol, olefins, formic acid). How long the CO2 remains bound by these uses depends on the product.
CCU technologies are essentially based on two steps isolated from each other: the separation step and the conversion step. Although the overall efficiency of the CCU process depends on the combination of these steps, CO2 capture and utilization have so far mostly been researched independently of one another. This circumstance has led to the fact that separation systems have already been developed on an industrial scale, while utilization or conversion technologies are still at various stages of development.
GIG Karasek therefore does not focus its research projects on CO2 capture, but on the development of innovative processes for CO2 utilization. The main focus here is on electrochemical CO2 conversion, which we will discuss in more detail below after presenting currently researched conversion pathways.
.png?width=600&height=220&name=CCU_Industrie_en%20(1).png)
Figure 1: CO2 capture from point sources and subsequent utilization of CO2 via various conversion pathways (self-representation).
2. CO2 utilization: conversion pathways and their technological maturity
Since CO2 is a thermodynamically stable compound, the conversion of CO2 usually takes place through a catalytic process with additional input, e.g. from renewable energy sources. Below is an overview of the main recycling technologies currently under investigation, including their technological readiness level (TRL):
2.1. Thermochemical CO2 conversion (TRL 5–9)
Thermochemical CO2 conversion uses catalysts and a combination of heat and pressure to convert CO2 into valuable products. With a technology readiness level between 5 and 9, this is currently the most mature technology for CO2 conversion. Challenges with this reaction pathway include reversibility and thermodynamic limitations of reactions.
2.2. CO2 mineralisation (TRL 4–8 concrete ingredients, TRL 7–8 concrete curing)
CO2 mineralisation processes are based on the reaction of metal cations (e.g. Mg, Ca, Fe) with CO2 to form solid and stable carbonate minerals and thus permanently store CO2. The resulting carbonates are potentially versatile, for example as fillers, cement additives or for land reclamation projects. CO2 mineralisation will nevertheless continue to make only a small contribution towards the decarbonisation of industry; it will not be able to offset the CO2 balance sheet on its own.
2.3. Electrochemical CO2 conversion (TRL 4–8 C1 products, TRL 1–3 C2+ products)
2.4. Bioelectrochemical CO2 conversion (TRL 1–3 one-step, TRL 4–7 two-step)
In the bioelectric conversion of CO2, the processes of electrolysis and fermentation are combined. For CO2 conversion, microorganisms are used that can reduce CO2 to methane in a one-step or two-step process. Hydrogen is produced in the first step of the two-step process. Then, in a second step, this is fed together with CO2 to a bioreactor containing anaerobic methanogenic bacteria for methane production. The one-step process is still poorly developed. The two-step process is close to commercialisation, but the process is rather slow compared to the electrochemical system.
2.5 Photocatalytic CO2 conversion (TRL 1–3)
The photocatalytic conversion of CO2 is currently still at the research stage. Using additives, a new raw material base is to be developed by making CO2 usable as a raw material for chemicals in a solar-powered recycling process. The targeted products are methane, synthesis gas and hydrocarbons.
2.3 Plasma catalytic CO2 conversion (TRL 1–3)
Plasma catalysis technology is another novel approach to CO2 utilization. In this process, a weakly ionised plasma, which is very reactive, is generated by supplying electricity. The conversion of CO2 into basic chemicals, such as methanol and ethylene, is activated by free electrons contained in the plasma. However, plasma chemistry is very complex and a better understanding of plasma reactions and interactions between the plasma and catalyst is needed.
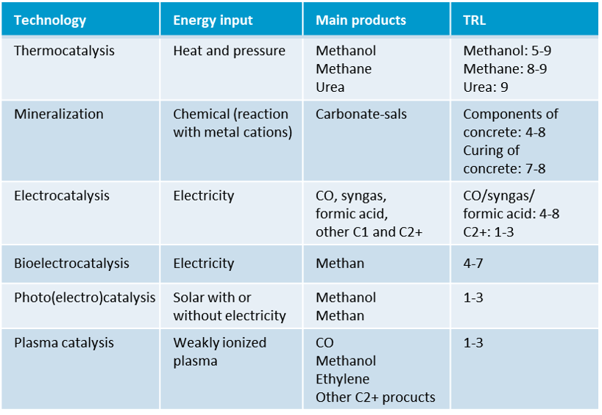
Table 3: CO2 conversion technologies, including the technical readiness level (TRL), prepared according to 1.
3. Electrochemical CO2 conversion as the way forward
From the range of utilization methods presented, the electrocatalytic or electrochemical conversion of CO2 (eCO2R) is gaining increasing interest in the scientific community. The following factors in particular are key to this:
- One-step conversion: The process enables the one-step conversion of CO2 into C1, C2 and even C2+ products (complex hydrocarbons). The electrochemical reduction of CO2 thus also produces products containing two or more carbon atoms per molecule (e.g. ethanol CH3-CH2-OH) in just one conversion step.
- Range of products: The electrochemical process therefore potentially allows the production of a wide range of products.
- More flexible use of electricity: Surplus renewable energy can be stored in the form of chemical energy and used again when needed. This enables a more flexible use of electricity (i.e. demand-side management).
Compared to other technologies, electrochemical CO2 reduction offers a number of significant advantages, which are discussed in more detail below.
4. Electrochemical CO2 conversion in comparison
Electrochemical reduction is an attractive technology because it can be carried out under mild conditions (near room temperature and ambient pressure) in aqueous solutions, using water instead of molecular hydrogen (H2) as the hydrogen source. Furthermore, electrochemical conversions are more attractive than other recovery technologies for several reasons, both in terms of energy efficiency and cost:
- Controllability: The main advantage of the CO2 reduction reaction (CO2RR) is the possibility to precisely control the conversion process (production of the desired products) through electrolysis parameters (potentials applied to the cathode, current density and electrolysis time), reaction temperatures and other parameters.
- High efficiency and scalability: The electrochemical conversion systems are modular, compact, highly efficient, easy to operate, demand-driven and scalable (i.e. the components of the electrochemical reactions are easy to use on a large scale).
- Use for fuels: The products obtained from chemical synthesis or energy storage can be used for fuels.
- Recyclability of components: The components of the electrolysis baths can be completely recycled, so that the total consumption of reagents can be minimised.
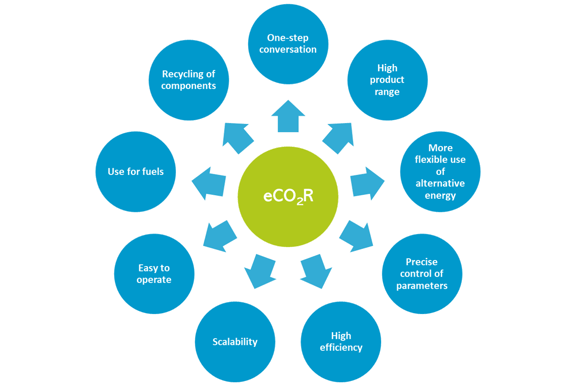
Figure 2: Advantages of electrochemical CO2 conversion (self-representation).
The previously mentioned alternative CO2 utilization technologies have some disadvantages compared to electrocatalytic conversion, which make them less attractive from GIG Karasek's point of view: 2
- Thermochemical CO2 conversion requires high pressure and high temperatures, which results in corresponding costs. The method is also not sustainable due to the use of a non-renewable energy source.
- The main disadvantage of the bioelectrocatalytic process is its high cost. Currently, biological methanation is even more expensive than thermochemical methanation, which is very cost-intensive anyway.
- The photocatalytic process is not possible for large-scale implementation and night-time operation due to the lack of solar radiation. The process is also less user-friendly and product isolation and separation is comparatively difficult.
- Plasma catalysis technology as another novel process is physically very complex and requires more research to fully understand the process.
For the reasons given, GIG Karasek relies on electrochemical CO2 conversion as a recycling technology. Below we provide a brief insight into the strategy pursued by GIG Karasek on this path.
5. Focus on formic acid and carbon monoxide
As already mentioned, electricity is applied in the electrochemical conversion of CO2 in order to use CO2 as a raw material for chemical syntheses. Depending on the catalyst materialused, the reaction pathway, the amount of CO2 feedstock and the energy, there are a variety of eCO2R products that can be produced.
GIG Karasek has selected formic acid (HCOOH) and carbon monoxide (CO) as two of the most promising products for the application of CCU technology:
- Formic acid is a bulk chemical that is of great interest for energy storage (formic acid fuel cells) and as a base material for the chemical industry.
- Carbon monoxide can be used in combination with hydrogen (H2) to produce synthesis gas and as a chemical base material.
Furthermore, CO and formic acid record the highest market price per electron used, although the market price is lower than for other products such as methanol and ethanol. They are therefore good candidates for the development of CCU technologies.
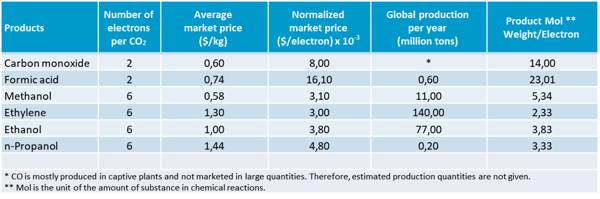
Table 4: Electrochemical CO2R products adapted from 3.
6.Innovative research project: direct electrolysis of CO2
GIG Karasek is currently involved in several research projects dealing with innovative approaches to the electrochemical conversion of CO2 into valuable materials. We have already presented the research cooperation with the Johannes Kepler University Linz (JKU Linz) on CO2 utilization based on the so-called "Dream Reaction".
As part of another project for the direct electrolysis of CO2, GIG Karasek is developing an expanded CCU system based on a new type of electrolysis cell in cooperation with three companies from Upper Austrian and the University of Innsbruck.
The aim of the project is to optimise the energy/cost ratio of CO2 capture and utilization of flue gases by means of direct CO2 electrolysis, by eliminating intermediate steps such as thermal desorption and product separation or processing. The capture and electrochemical conversion of CO2 thus no longer takes place in two separate steps, but is integrated into one unit.
The innovative technology is based on a symbiotic CO2 absorption and electrolysis cell that significantly streamlines the process and increases energy efficiency. This mini-plant system serves as the basis for the next scale-up steps for industrial research with the aim of establishing CO2 utilization as a future business sector and taking an innovative step in waste gas purification.
GIG Karasek contributes its expertise in industrial plant construction with regard to separation technology and cell periphery construction with its production sites in Attnang-Puchheim and Gloggnitz and a planning office in Graz.
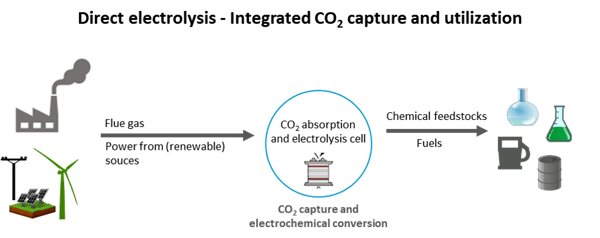
Figure 3: In direct electrolysis, CO2 capture and conversion to valuable products takes place in just one step. Ideally, the process is fed from renewable energy sources (self-representation).
7. Challenges in electrochemical CO2 reduction in industry
The most important bottleneck on the way to industrialising the electrochemical CO2 reduction process is system integration and optimisation at system level.
The reactor cell with integrated catalyst forms merely the basis of the technology. The greater challenge is to optimally integrate the reactor cell into the overall system and make it operational. Selectivity, activity and stability of catalysts are key parameters in this respect:
- Activity describes the performance of a catalyst at a given energy input.
- Selectivity defines whether and how many contaminating by-products are generated.
- Stability indicates how efficient a catalyst is in the long term.
An exclusive focus on high selectivity, activity, stability, costs or scalability does not lead to the desired results. Ultimately, achieving the optimum requires a compromise between all parameters.
Another challenge in the industrialisation of eCO2R is the current density. The industrial production of value products from CO2 requires an increase in current density from milliamperes (mA) to several amperes (A). This is still a challenge for most systems.
To work out the best possible option, it is necessary to realise this technology on a large scale in pilot plants or demonstration electrolysers. Within our pilot plant and our research projects, we therefore investigate and improve every parameter. The focus here is on the type of electrocatalyst, the morphology of the electrocatalyst, the electrolyte composition and the process conditions.
8. Outlook for the CO2 utilization industry
The economic feasibility of CCU concepts depends not only on technological readiness, but also on the creation of political framework conditions. These include, in particular, carbon tax policies, government support for technology development, and incentives for environmentally friendly production pathways.
Industrial eCO2R is not yet competitive compared to fossil fuel alternatives for the chemical industry and energy storage. However, as soon as the electrical energy required for conversion can be fed from renewable energy sources, competitive production is possible. This is especially true in view of enormously rising prices for energy from fossil sources.
Of course, the electrification of the chemical industry is a challenging path in terms of economic competitiveness with industries based on fossil fuels or non-electrochemical pathways. Nevertheless, GIG Karasek is convinced that the development of large-scale electrochemical technologies (e.g. eCO2R) that run on emission-free electricity is necessary in the future, given the enormous environmental impact and the unavoidable CO2 emission costs.
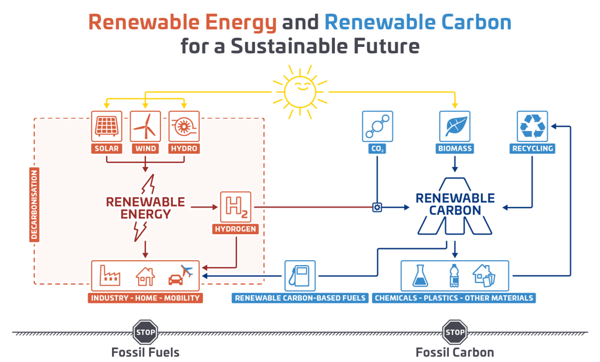
Figure 4: CCU technologies in the context of a sustainable chemical industry, adapted according to © nova-institute.eu.
9. Conclusion: CO2 utilization as a new business sector
CO2 can be made usable as a raw material in the medium term through innovative technologies; for example, for plastics, building materials or fuels. Decisive advantages for CO2 utilization as a future business sector are offered by technologies that can use electrical energy generated from renewables directly for CO2 conversion. Electrochemical CO2 conversion at moderate process temperatures is a particularly efficient and environmentally friendly method for this. Research and development of this CO2 conversion pathway must therefore take place now in order to be prepared for the future.
Find out more about why investing in this market can make sense right now in our article “CO2-Utilization: 5 reasons why companies should participate now".
1 Nishikawa, E. (29 April 2022). CO2 conversion & utilization pathways: Techno-economic insights. PreScouter. https://www.prescouter.com/2022/04/co2-conversion-utilization-pathways/ (last accessed 27/09/2022)
2 Samanta, S., & Srivastava, R. (2020). Catalytic conversion of CO2 to chemicals and fuels: the collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride. Materials Advances, 1(6), 1506–1545. https://doi.org/10.1039/d0ma00293c
3 Mohsin et al., Economic and Environmental Assessment of Integrated Carbon Capture and Utilization, Cell Reports Physical Science (2020), https://doi.org/10.1016/j.xcrp.2020.100104
4 CO2: Raw material instead of pollutant. Cleantech-cluster.at. https://www.cleantech-cluster.at/news-presse/detail/news/co2-rohstoff-statt-schadstoff (last accessed 27/09/2022)
5 Steeg, D., Renewable energy and renewable carbon for a sustainable future − graphic. Renewable Carbon Publications. Last accessed 18 October 2022, from https://renewable-carbon.eu/publications/product/renewable-energy-and-renewable-carbon-for-a-sustainable-future-%e2%88%92-graphic/



